Saturday 18th July 2020
Geopark terras de Cavaleiros - zoom meeting July 15th 2020
Earlier this year club secretary Dr Chris Simpson went on a tour to the Geopark Terras de Cavaleiros Portugal which was organised by Chris Darmon (Geo-supplies www.geosupplies.co.uk). The talk is based on photographs taken on the tour and information supplied by Chris Darmon. Further details of the Geopark can be found on his website
The Geopark is located in north-eastern Portugal on the border with Spain. The Iberian Peninsula has a lot of interesting geology; but this area is very special because in addition to the background autochthonous ( formed in it’s present position) rocks, there are two separate assemblages of allochthonous (formed away from present position) rocks, i.e. rocks which have been formed elsewhere, and then moved to their present site by large-scale thrusting.
The photograph below shows a portion of a 2.5m map of the Geopark in the main Geopark building. The allochthonous rocks are the roughly circular cluster near the bottom of the photo divided into two halves by a nearly horizontal fault line.

The bottom half is the superior allochthonous group which represent a piece of continental crust. The top half is the inferior allochthonous group which represent a piece of oceanic crust. Both these assemblages were emplaced at differing times during the compression and eventual disappearance of the Iapetus Ocean between about 450Ma and 350Ma.
Apart from the main building in Macedo de Cavaleiros with plentiful rock samples, diagrams of the geology and information sheets, each site in the park has an information board in Portuguese and English. What follows is some examples of the range of geology available in this geopark.

parasitic folding in Ordovian quartzite. Compare the undeformed top stratum in the distance with the folds in the foreground.

Flasergabbro – a distinctive rock type which is a partially metamorphosed gabbro from the oceanic crust allochthen

a remarkable contact. The bottom layer is amphibolite, i.e. metamorphosed basalt from the oceanic crust. The top layer is gneiss, which is metamorphosed continental crust rock. (One post of an information board is seen at top left.)

an exposure of granulite – a rock type which is found below the Conrad Discontinuity and is thus a very rare finding. This site is of international importance. (A discontinuity is a boundary between two rock layers of significantly different density. The Conrad Discontinuity marks the lower boundary of continental crust.)

A coarse tuff, the product of an andesitic volcano, in which there are several pale clasts, all showing the same orientation. This appearance suggests that this tuff was not the usual aerial deposition, but came from a lahar of tuff cascading down the side of a volcanic cone.

an old quarry. This is an isolated limestone deposit showing partial metamorphism to marble. It is poor quality stone – but as it is the only deposit of its type for hundreds of miles around, it was worth quarrying in the 19th century. There are no fossil corals, so this was likely to be an isolated stromatolite reef.
Friday 6th March 2020
At the last meeting Dewi Roberts gave a very interesting talk on fluvial geomorphology. The interest was enhanced by the use of underwater photography within the river which highlighted the processes occurring on the river bed.
Streams begin with water added to the surface which will run off down the steepest slope.Subsurface water and ground water also add water to the system. Erosion commences with the creation of small rill channels which coalesce and deepen into channels. The channels lengthen upslope by headward erosion. Dewi gave a good explanation of the latter by showing a clip of headward movement in beach sand. Over time channels merge and tributaries join a large trunk system. The types of drainage pattern that are observed are dependent on the type and structure of the rock and how easily the rock can be eroded.
He went on to discuss the load that is carried by a stream which can be divided into three types:
suspended load which are those particles which are carried along with the water in the main part of the stream, the size of which is dependant on their density and the velocity of the stream.
bed load which are the coarser and denser particles which move along the bottom by the process of saltation.
dissolved load which are the ions dissolved within the water.
The maximum size of particle that can be carried is called the competence and this varies with the sixth power of velocity. The capacity is the maximum stream load that a stream can move over a given time and varies greatly over time. So that the most work a stream does will occur during floods when stream velocity and discharge are greatly increased.

Pot-hole erosion in the river Marteg
As a stream moves down from it’s head to the mouth of the estuary then it’s discharge increases as does it’s cross sectional area and it’s velocity ( on average). This latter idea of velocity increase had us all wondering but it seems that the broad lowland waters have a much greater discharge and hydraulic radius and the waters flow much more freely. Hence the increase in velocity.

Severn Break-it's-Neck waterfall in the upper reaches of the river Severn
Using photos of local rivers we looked at the evolution of the stream valley from it’s youthful v-shaped valley with little or no flat land next to the stream. As the stream continues downstream it begins to erode more laterally than vertically and the valley widens and the stream begins to meander causing erosion on the outer portion of the meandering bend where velocity is highest and sediment will be deposited on the inner bend where velocity is low. If erosion continues on the outer bend then a meander may eventually be cut off leaving an oxbow lake.

Meanders in the river Severn
Stream deposition occurs whenever there is a change in velocity. Velocity varies along the length and wherever there is a lowering of velocity some sediment will come out from suspension and be deposited.For example if the discharge is suddenly increased, as it might be during a flood, the stream will overtop its banks and flow onto the floodplain where the velocity will then suddenly decrease. This results in deposition of such features as levees and floodplains. If the gradient of the stream suddenly changes by emptying into a flat-floored basin, an ocean basin, or a lake, the velocity of the stream will suddenly decrease resulting in deposition of sediment that can no longer be transported. This can result in deposition of such features as alluvial fans and deltas.
This was a very enjoyable talk.
The next meeting will be on Wednesday 18th march when Prof.Ian Stimpson will give a talk on Geothermal Energy in the UK
Monday 20th January 2020
The meeting on January 15th consisted of the AGM followed by an auction of books and maps kindly donated by Prof. Bill Fitches. Bill Bagley (Chair) then gave a talk on Chalcedony.
Chalcedony is one of the multitude of varieties of quartz (formula
SiO2). It is microcrystalline or cryptocrystalline, often botyroidal and often crystallises in layers in voids in the rock.
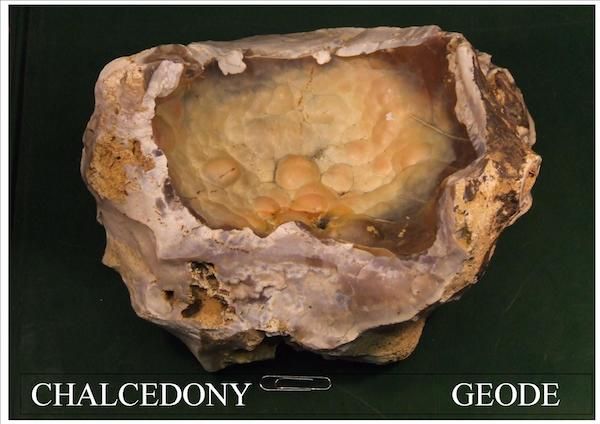
chalcedony from Normandy France
Bill introduced the talk with an old slide showing the range of
varieties of quartz which was now outdated as a new variety, moganite,
had been discovered in recent years.

moganite by Bergminer creative commons
Moganite has the same composition as quartz, but its crystal form is monoclinic, whereas quartz is trigonal. It is now known that chalcedony often comprises a proportion of moganite. X ray fluorescence or spectroscopy is required to distinguish between them.
Bill took us on a grand visual tour of the colourful varieties of
chalcedonite, agate, opal, onyx and jasper. A real mineralogical
treat!

banded chalcedony from Lincolnshire fens
Thursday 14th November 2019
At the last meeting, 16th October, committee members Michele Becker and Bill Bagley gave a talk on the Physical Properties of Minerals in Relation to their Internal Structure.
Michele began with a definition of a mineral i.e. a substance with the following criteria: Naturally occurring; solid at standard temperature and pressure ( with the exception of mercury); a specific chemical composition which varies within a very narrow range and has an ordered 3D array of atoms/molecules within it’s structure.
She then went on to give a basic overview on types of chemical bonds as the type and strength of these bonds along with the chemistry and geometrical arrangement of atoms of a mineral will determine it’s physical properties.
This was followed by a brief look at crystal structure. Crystal structure is a highly ordered repeatable structure. The simplest repeating unit in a crystal is called the Unit cell and each Unit cell is defined in terms of lattice points which is repeated in 3D to give the lattice structure. Symmetry and form of a crystal are determined by the shape of the unit cell. Each unit cell contains one or more different kinds of atoms joined to each other by chemical bonds. All unit cells have six sides - 3 sets of parallel faces though not necessarily perpendicular to each other. Basically there are 7 kinds of unit cell which differ in the relative lengths of their edges and the angles between them. These are cubic, hexagonal, trigonal, tetragonal, orthorhombic, monoclinic, triclinic.
This was followed by a look at some physical properties and how they are determined by mineral structure.
Habit and form - habit is the tendency for a mineral to repeatedly grow into characteristic shapes and is influenced by the atomic structure and the environment in which it grows.
Form - is similar to habit and may be defined as a solid crystalline object bounded by a set of flat faces related to one another by symmetry.
Hardness - is the ability of a mineral to be scratched. The test used is the Mohs hardness test which is a non-linear, ordinal scale of hardness. In this scale talc is the softest whilst diamond is the hardest.
The hardness of a mineral is dependent on the packing pattern, the electrostatic charge on the ion and the distance between. The presence of water also changes hardness.
An example can be seen between diamond and graphite both consisting of carbon. Diamond measures 10 on Mohs scale whilst graphite is 1 on the scale. This is due to their internal structure. In diamond each carbon atom is bonded to four others in a tetrahedral shape and forms a highly interlocking structure. In graphite each carbon is linked to three other carbons in hexagonal sheets which look like chicken wire. These sheets are separated by Van der Waal bonds which are very weak.
Cleavage - Is a plane of structural weakness along which a mineral is likely to split. This is due to either weaker bond strength or greater lattice spacing. Cleavage is usually parallel to potential crystal faces. Not every mineral shows cleavage eg. quartz has no cleavage it only fractures which is related to a greater bond strength in quartz. Some minerals have one direction of cleavage like biotite and muscovite mica whilst others may have two ( eg.feldspars), three (eg. calcite) and even four ( fluorite)
Cleavage can be described as: perfect, distinct, difficult, imperfect. or indistinct.

Calcite has three directions of cleavage not at 900

Fluorite has four directions of cleavage
Density/specific gravity - is dependent on the chemical composition and crystal structure. Heavier atoms/molecules, closer the packing and stronger the bond strength the greater the density. eg. diamond has a SG of 3.5, graphite 2.5.
Also in isostructural compounds there is a direct relationship to the mass of atoms for example:
Olivine - varies from forsterite - MgSiO4 - SG 3.22 to fayalite- FeSiO4 - SG 4.41
In the second half of the talk Bill went on to describe colour in minerals. This commenced by a short introduction to visible light.
White light is defined as the complete mixture of all of the wavelengths of the visible spectrum and forms just a small part of the electromagnetic spectrum.The properties of light include refraction, reflection, interference and diffraction.
A rainbow (or a prism) splits sunlight (white light) into its component colours because it bends different colours ( i.e. different wavelengths of light) by different amounts. Shorter wavelengths are bent more than longer wavelengths, so blue light is bent more than red.
How we see colour is dependent on the fact that some wavelengths are absorbed by an object whilst others are reflected. These reflected wavelengths enter the eye of the viewer and are interpreted as colour. For example a leaf appears green as all colours of the spectrum except green are absorbed, green is reflected and perceived by the viewer.
He then went on to discuss the causes of colour in minerals.
An achromatic mineral is one without colour eg. white of calcite. An Idiochromatic mineral is one in which the colour is directly related to the elements present in it’s chemical composition for example the manganese carbonate rhodochrosite is always pink.

Rhodochrosite
An allochromatic mineral is one that is coloured by impurities that are not part of it’s chemical composition for example the purple of amethyst is due to the presence of small amounts of iron in quartz.

Amethyst
The elements that lead to the production of colour are usually those ions from the first row of the transition elements ( Ti to Cu). These ions have electrons in the five 3d orbitals. In the crystallographic sites found in minerals, the 3d orbitals split into different energies. Visible light interacts with these electrons and causes them to be excited to higher energy orbitals. The wavelengths that cause these transitions are subtracted from the incident light resulting in colour.
Charge transfer is another means of eliciting colour. This is the result of charge change for example between two metals. These metals can exist at different valence states eg. iron and titanium. The absorption of light energy causes an electron to transfer from one ion to the other; it then returns to the original ion. For example, on absorption of light, the pair iron +2/titanium +4 becomes iron +3/titanium +3, resulting in the blue colour in sapphire.
Hole centres is a third means of colour generation. This can be illustrated by smokey quartz. In quartz some silicon ions which have a valence state of +4 may be replaced by aluminium ions with a valence state of +3. electrical neutrality will be maintained by the presence of sodium or hydrogen ions, although this will weaken the forces on the electrons of oxygen atoms in the structure. An input of external radiation can thus remove an electron from the oxygen leaving a hole, and now different energy levels become available to the remaining unpaired electron on the oxygen. Thus clear quartz will become smokey brown in colour. If the crystal is heated the electron can return to its original location and the colour generated by the above will be removed.

Smokey quartz
Saturday 19th October 2019
Evening talk by Dr. Keith James September 18th 2019. The talk was entitled, "Not Written in Stone:Plate Tectonics at 50"
Dr. Keith James is a consultant geologist and fellow of the Institute of Geophysics and Earth Sciences at Aberystywyth University in Wales. He has worked with Shell all over the world - and later with Conoco.
Dr James commenced his talk by saying that published Plate Tectonics teaching is complacent. It should adapt to emerging data, including multiple working hypotheses, and enable students to think.
He then went on to describe a number of dicoveries that he suggests should put the theory of plate tectonics into doubt.
His ideas are controversial and many will disagree so the following link is to a PDf of his ideas and you can come to your own conclusions.
[https://mid-wales-geology.s3.eu-west-2.amazonaws.com/news/NCGT+K.James.pdf]

















